Welcome to compligen.ai

At compligen.ai, we offer a Power Al-driven e-Validation platform, cGen, designed to streamline Computer System Validation (CSV) and Computer Software Assurance (CSA) for regulated industries, including Pharma, Biotech, Food, OTC, Dietary Supplements, Tobacco, Cosmetic, and Medical Devices (compligen.ai).
What Is cGen?
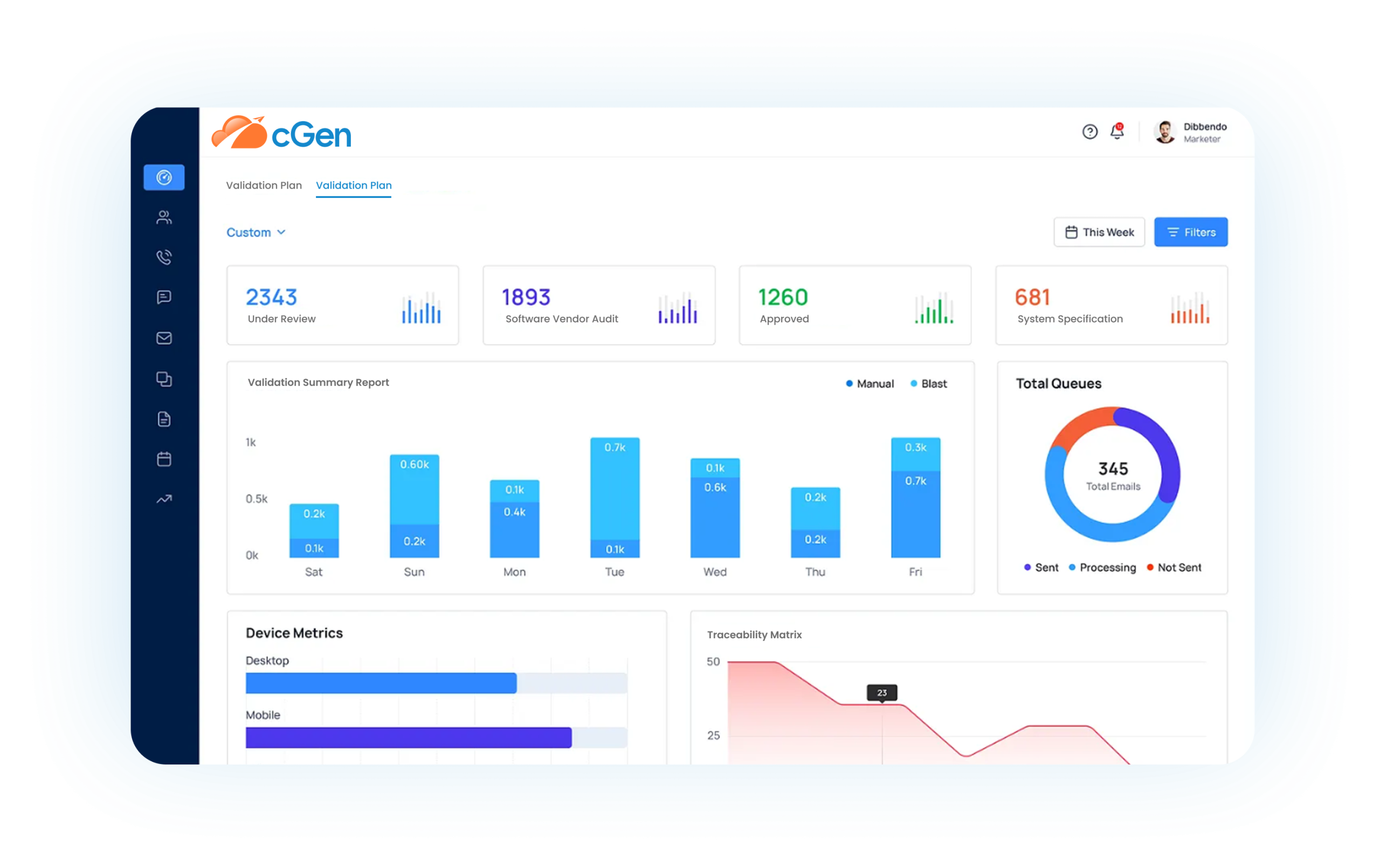
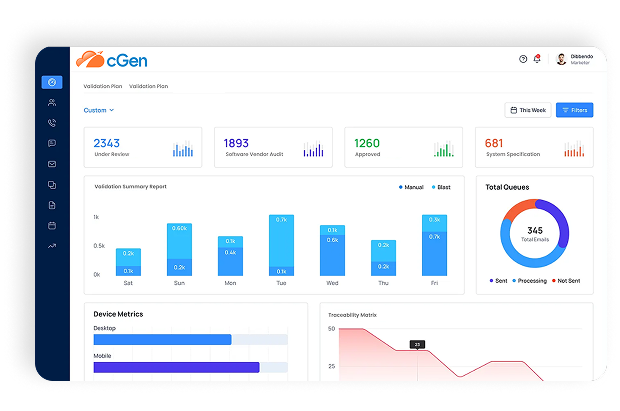
cGen is compligen.ai’s next‑generation electronic validation platform, built on over 35 years of FDA CSV experience. It uses AI to automate CSV and CSA activities—replacing outdated paper or hybrid validation methods—with robust support for compliance to FDA, EMA, MHRA, GAMP 5, and ISO standards (compligen.ai).
Benefits of cGen
Streamlined e‑Validation:
Automate your URS, FRS, IQ/OQ/PQ, traceability matrices, test plans and reports—guided by intelligent validation logic and built-in regulatory knowledge.
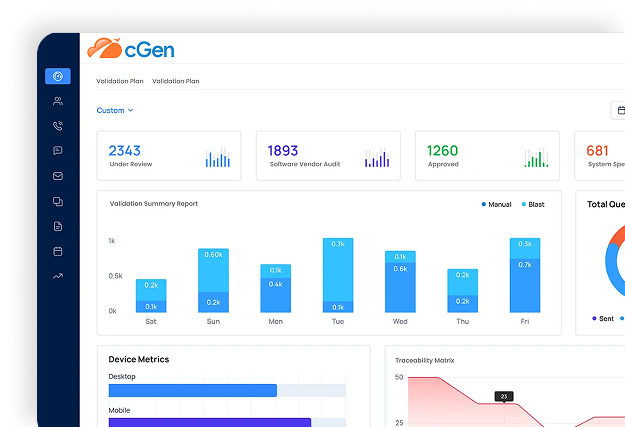
Risk‑Based, AI‑Powered CSA Approach:
Align system risk with patient safety, product quality, and data integrity.

Key Features at a Glance
Feature
- Automated Validation Documents
- Traceability Matrix Automation
- Intelligent Software Change Control
- AI Validation Assistant
- Automatic Periodic Reviews
- System Retirement
Benefit
- Generate essential validation deliverables seamlessly.
- Link requirements through test plans and results with full auditability.
- Track system changes, approvals, and validation status lifecycle‑wide.
- Export reports in both human‑readable and machine‑readable formats.
- Receive contextual recommendations, risk insights, and guidance tailored to your system configuration.
Who Can Benefit?
Pharmaceutical, biotech, and medical device manufacturers
GxP‑regulated SaaS and LIMS providers
CROs, CMOs, and contract labs
Validation, Quality, and IT teams managing CSV/CSA compliance


Why Choose cGen
Cut validation cycle time.
1

2
Ensure full end‑to‑end compliance.

Minimize manual effort and error.
3

4
Improve documentation consistency and accuracy.

Be audit‑ready on‑demand with minimal prep.
5
Seamless Integrations
- Ticketing Tools: Jira, ServiceNow
- Document Management: SharePoint, Veeva
- Quality Systems: QMS, LIMS, ERP, MES
What people are saying

We transitioned to their cloud platform in under a week. The performance boost and ease of use were game-changers.

Jennifer Dilly

We transitioned to their cloud platform in under a week. The performance boost and ease of use were game-changers.

Mark

We transitioned to their cloud platform in under a week. The performance boost and ease of use were game-changers.

Mark
FAQ
About the Platform
What is compligen.ai?
What is cGen?
cGen is compligen’s core e-validation engine. It automatically generates validation deliverables such as Validation Plans, System Requirement Specifications, IQ/OQ/PQ protocols, Traceability Matrices, and Validation Summary Reports, saving time and reducing compliance risks.
Features & Functionality
What validation deliverables can cGen generate?
cGen can produce the following deliverables automatically:
– Software Vendor Audits
– Validation Plans
– System Requirements Specifications (SRS)
– Installation Qualification (IQ)
– Operational Qualification (OQ)
– Performance Qualification (PQ)
– Traceability Matrices
– Validation Summary Reports
Can compligen.ai support both CSV and CSA approaches?
Yes. The platform supports both traditional CSV and the FDA’s risk-based CSA approach,allowing organizations to align with the latest regulatory guidance.
Does compligen.ai provide audit trail and electronic signatures?
Yes. All validation documents generated via cGen are 21 CFR Part 11 compliant, with full audit trails, electronic signature validation, and version control.
Compliance & Security
Is compligen.ai FDA 21 CFR Part 11 compliant?
Yes. compligen.ai ensures compliance with FDA 21 CFR Part 11, EU Annex 11, and related global GxP regulations for electronic records and signatures.How does compligen.ai ensure data integrity?
The platform enforces ALCOA+ principles (Attributable, Legible, Contemporaneous, Original, Accurate, plus Complete, Consistent, Enduring, and Available). Data is encrypted, access-controlled, and fully traceable.How often are validation deliverables updated?
Deliverables are updated automatically whenever requirements or system changes occur, ensuring continuous compliance and audit readiness.
Implementation & Integration
How quickly can we implement compligen.ai?
Most organizations can implement compligen.ai within weeks, as the platform is cloud-based and requires minimal setup.Does compligen.ai integrate with existing systems?
Yes. compligen.ai can integrate with quality management systems (QMS), ERP platforms, LIMS, MES, and other regulated applications.Can compligen.ai be used for vendor audits?
Yes. The platform includes automated Software Vendor Audit templates and reporting features to support supplier qualification and oversight.Does cGen require software validation?
Yes, cGen is a GxP SW application that is used to create and maintain validation records, which are considered GxP records. compligen offers a SW validation kit that can be executed to validate your instance of cGen.
Support & Services
Do you provide training for users?
Yes. We provide comprehensive onboarding, training sessions, and ongoing customer support to ensure successful adoption.What industries can benefit from compligen.ai?
Life sciences (pharmaceutical, biotech, medical device), healthcare, food & beverage, and any industry operating under FDA or GxP regulations.How does compligen.ai reduce compliance risk?
By automating documentation, enforcing standardized processes, and providing audit- ready records, compligen.ai minimizes human error and ensures continuous inspection readiness.What’s Next?
How to Get Started